
New platforms deflect the financial impact of cancer tests
January 18, 2024What is MRD Testing?
Minimal Residual Disease testing is like the elite special forces of cancer diagnostics. It doesn’t just tell you if cancer is there; it tells you how much, or little, is left after treatment. Traditional methods might give cancer the all-clear, but MRD testing goes deeper, detecting even the sneakiest of cancer cells hiding out at levels undetectable by standard tests.
Why is MRD Testing Important?
Precision in Prognosis: MRD testing is like having a high-precision radar. It can predict relapse risks and survival outcomes with much greater accuracy. Knowing if a patient is MRD-positive or negative helps doctors tailor treatment plans more effectively.
Treatment Decisions: It’s like having a roadmap in a dense forest. MRD testing guides oncologists in deciding whether to intensify treatment for those at higher risk or de-escalate for those at lower risk, avoiding unnecessary toxicity.
Monitoring Response: This is real-time intel on how well treatment is working. It’s like watching the enemy’s movements closely and adjusting strategies accordingly.
MRD vs. Traditional Methods
Traditional cancer tests are like old-school binoculars; they give you a broad view. MRD testing, on the other hand, is like a high-powered microscope. While traditional methods rely on imaging and physical examinations, MRD testing detects cancer at the molecular level. This means catching those few remaining cells that could potentially cause a relapse.
Impact on a Patient’s Life
Peace of Mind: Knowing you’re MRD-negative can be a huge relief. It’s like knowing the coast is clear after a storm.
Tailored Treatment: No more one-size-fits-all approach. MRD testing leads to personalized treatment, reducing the risk of over or under-treatment.
Early Intervention: If MRD testing shows that cancer cells are lingering or returning, it’s a signal to act fast, potentially stopping a relapse before it fully develops.
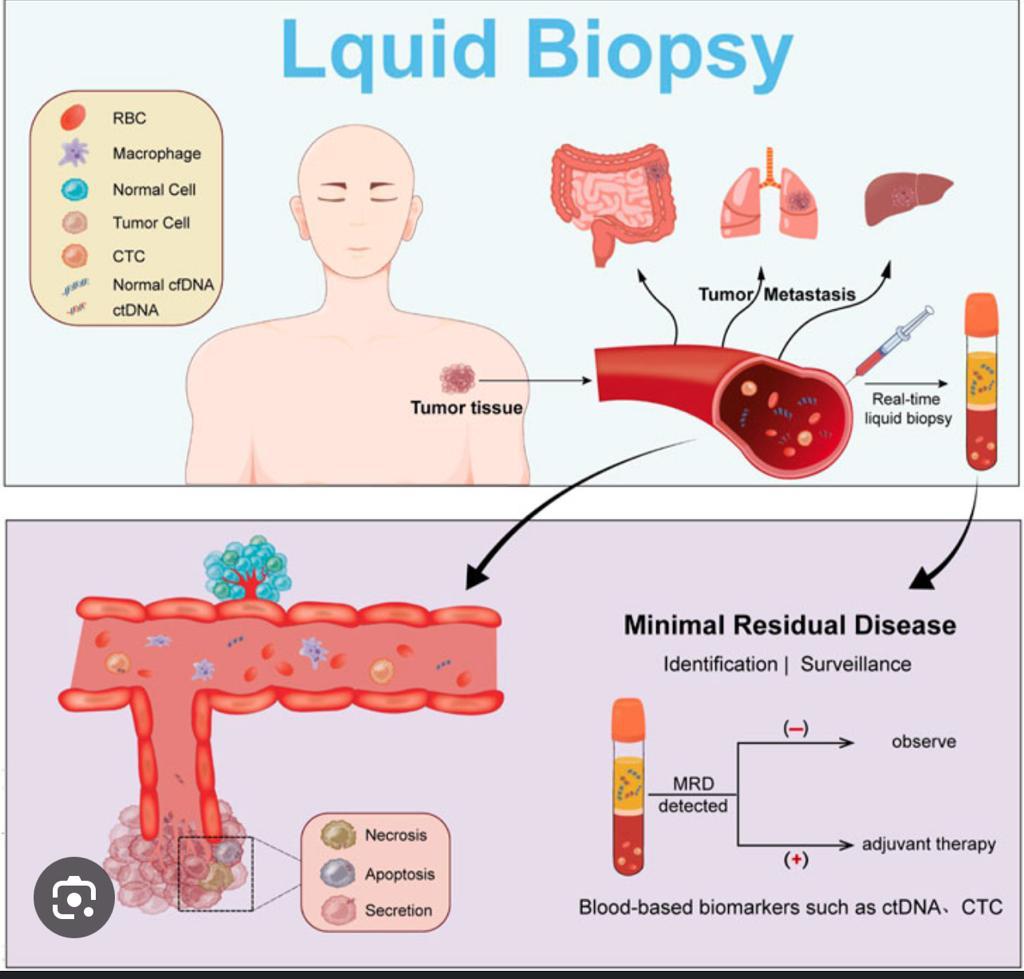
Key Factors of MRD:
- Higher Levels of MRD and Relapse Risk:
- Generally, higher levels of MRD after treatment are associated with a higher risk of relapse. Studies in various cancers, such as leukemia, lymphoma, and some solid tumors, have consistently shown this correlation.
- Time to Relapse:
- The time to relapse after treatment can be influenced by the amount of MRD detected. Patients with higher levels of MRD typically have a shorter time to relapse. In some cases, this could be within 6 months to a year.
- Quantitative Analysis:
- Some studies provide quantitative analysis. For example, in certain types of leukemia, patients with a certain level of MRD may have a relapse rate of over 50% within a year, compared to less than 10% for those with no detectable MRD.
- cfDNA as a Biomarker:
- The detection of cfDNA has been increasingly used as a non-invasive biomarker for MRD. High levels of cfDNA post-treatment are often indicative of a higher likelihood of relapse, but the exact percentages can vary.
- Individual Variability:
- It’s crucial to note that these statistics can vary greatly between individuals. Factors like genetic differences, the presence of other health conditions, and individual responses to treatment play significant roles.
TRACERx study:
The patient-specific ctDNA enrichment panels demonstrated impressive sensitivity and specificity, detecting variant fractions as low as 0.003% of the total DNA sample.
Analysis of 271 postoperative ctDNA samples taken periodically from 37 individuals without disease relapse showed that 269 of the samples—more than 99%—were MRD negative.
There was a single exception among the 26 patients with no evidence of clinical relapse, which was linked to an individual who had not yet undergone adjuvant radiotherapy, and that likely reflected delayed clearance of residual disease.
The other exception was a single false-positive result that occurred among the group of 11 patients with second primary tumors in the lung or elsewhere, thus reflecting the specificity of the ctDNA enrichment panel toward the primary tumor.
Among the 53 patients with NSCLC relapse, the TRACERx team found that some individuals, particularly those with non-adenocarcinoma histology, shed tumor DNA in blood prior to surgery. This finding proved relevant. ctDNA could be detected at or before relapse in 91% (38/42) of preoperative shedders versus 64% (7/11) of nonshedders. Notably, the median lead time prior to relapse was 164 days in shedders versus 22 days in nonshedders.
Conclusion
MRD testing isn’t just another medical test; it’s a critical tool in the fight against cancer. It brings a level of precision to cancer care that was previously unattainable, offering hope for better outcomes and more personalized treatment strategies. As we continue to advance in our understanding and technology, MRD testing stands as a beacon of progress in oncology.
Stay informed, stay ahead, and let’s keep fighting the good fight against cancer.



1 Comment
I may need to read this a couple of times to fully understand, due to my limited knowledge. Nevertheless, fascinating and admirable work Ramin.